Discovering New Precision Medicines
Building on an exceptional track record of innovation, this team brings a wealth of cross-functional expertise to successful rational drug design. We focus on pioneering best-in-class therapeutics to improve upon existing drugs with clear liabilities, as well as to create new breakthroughs for diseases where others have been unable to find solutions.
Established and emerging science will guide our plans, with an emphasis on rare disease patient populations with well-characterized biology, allowing us to move rapidly and create meaningful impact for patients.
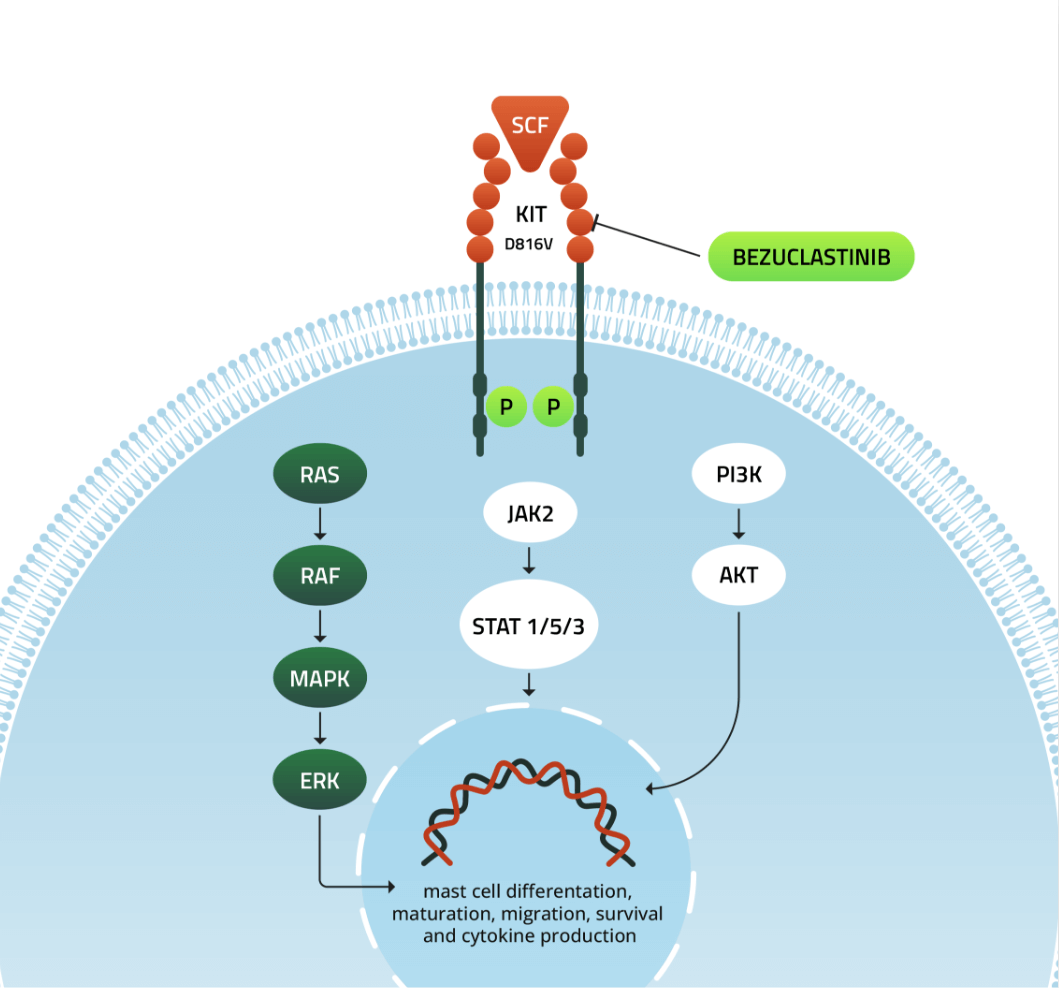
KIT mutations serve as driver mutations in up to 80% of gastrointestinal stromal tumors (or GIST) and over 90% of systemic mastocytosis (SM), a hematologic disease of clonal mast cells. Bezuclastinib is a novel type I tyrosine kinase inhibitor being developed as a potent and selective inhibitor of KIT A loop mutations, including D816V to address diseases driven by mutated KIT.
Bezuclastinib is a novel type I TKI that is being developed as a highly potent and selective inhibitor of KIT D816V and may avoid off-target toxicities to provide a better clinical profile. Bezuclastinib is currently being evaluated in three ongoing trials; two trials in Phase 2 development for Systemic Mastocytocis and a Phase 3 for GIST.
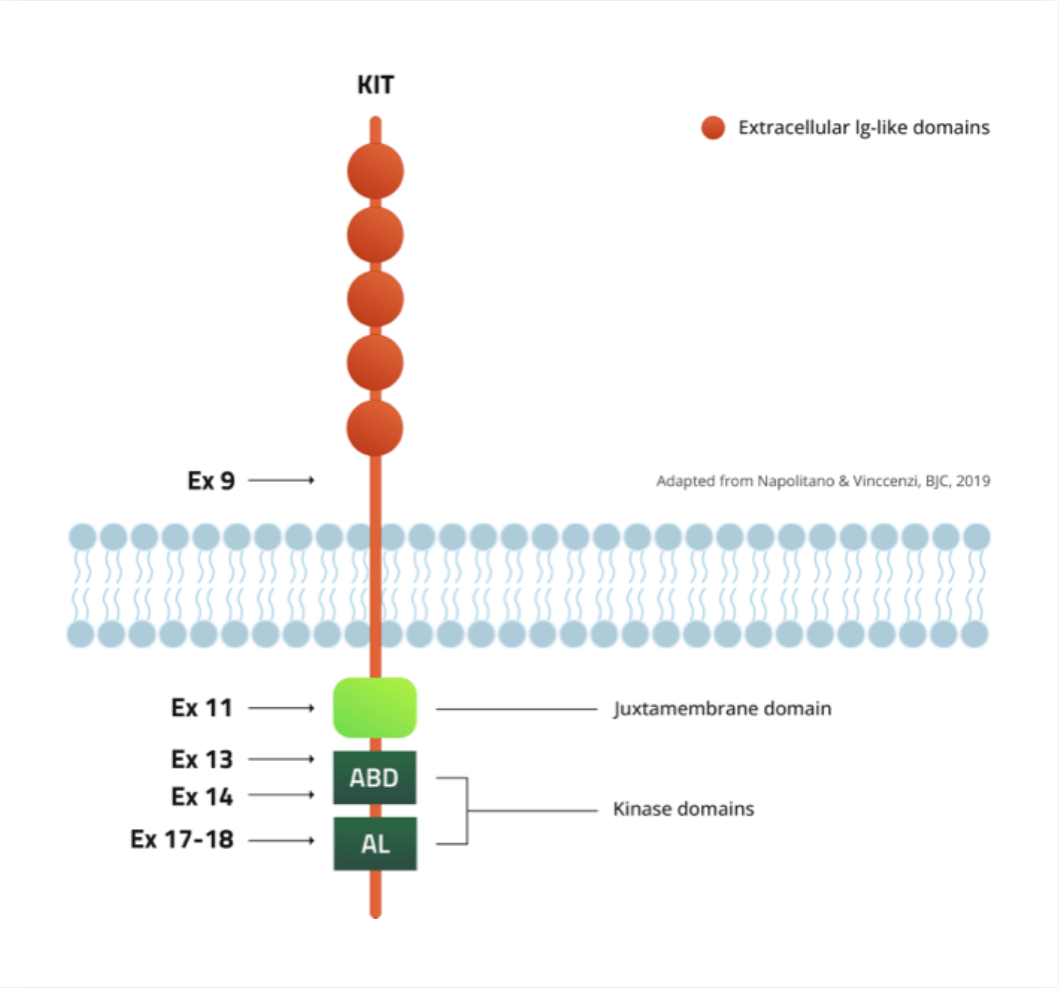
In GIST, patients often relapse after front-line imatinib treatment due to secondary mutations in ATP-binding domain (ABD) or Activation Loop (AL). Although second-line sunitinib is active against ABD mutations, it is not active against all AL mutations. Inhibitors targeting AL mutations, notably D816V (a common AL mutation in SM), have shown activity in the treatment of advanced SM, but off-target toxicities of available compounds may limit optimal clinical dosing.
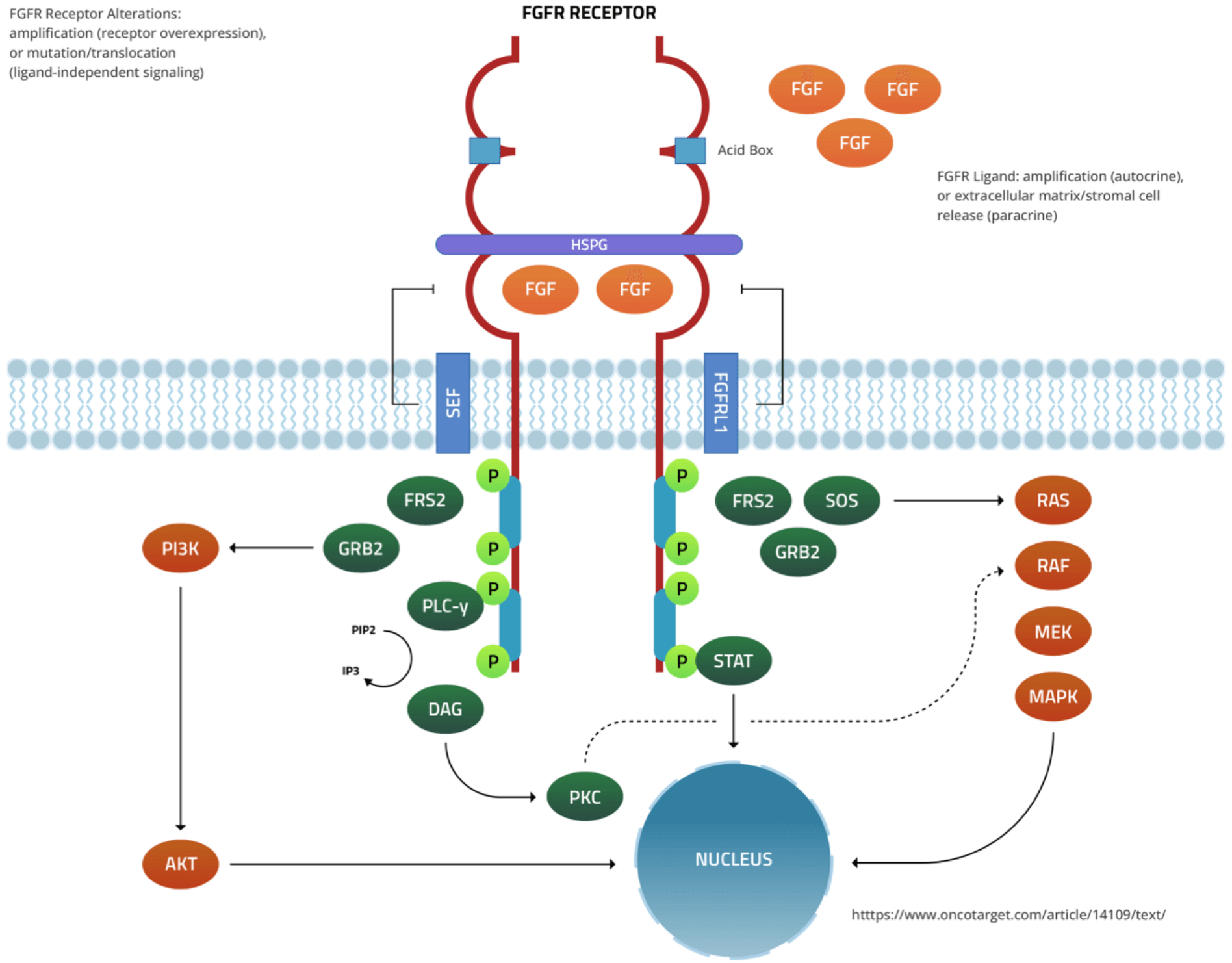
Inhibition Ligand Independent Signaling in FGFR 2/3 Altered Tumors is a Clinically Proven Approach
| wdt_ID | wdt_created_by | wdt_created_at | wdt_last_edited_by | wdt_last_edited_at | INDICATION | DATE | TITLE | JOURNAL/CONFERENCE |
|---|---|---|---|---|---|---|---|---|
| 2 | cmatranga@macdougall.bio | March 2024 08:48 PM | cmatranga@macdougall.bio | March 2024 10:52 PM | Non-Advanced Systemic Mastocytosis | February 2024 | American Academy of Asthma, Allergy & Immunology Annual Meeting | |
| 3 | cmatranga@macdougall.bio | March 2024 08:49 PM | cmatranga@macdougall.bio | March 2024 08:08 PM | Advanced Systemic Mastocytosis | December 2023 | American Society of Hematology Annual Meeting | |
| 4 | cmatranga@macdougall.bio | March 2024 08:50 PM | cmatranga@macdougall.bio | March 2024 08:20 PM | Non-Advanced Systemic Mastocytosis | December 2023 | American Society of Hematology Annual Meeting | |
| 5 | cmatranga@macdougall.bio | March 2024 08:51 PM | cmatranga@macdougall.bio | March 2024 08:22 PM | ErbB2 | December 2023 | San Antonio Breast Cancer Symposium | |
| 6 | cmatranga@macdougall.bio | March 2024 08:52 PM | cmatranga@macdougall.bio | March 2024 08:22 PM | PI3Kα | December 2023 | San Antonio Breast Cancer Symposium | |
| 7 | cmatranga@macdougall.bio | March 2024 08:52 PM | cmatranga@macdougall.bio | March 2024 08:23 PM | Gastrointestinal Stromal Tumors | November 2023 | Connective Tissue Oncology Society Annual Meeting | |
| 8 | cmatranga@macdougall.bio | March 2024 08:53 PM | cmatranga@macdougall.bio | March 2024 08:23 PM | FGFR2 | October 2023 | AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics | |
| 9 | cmatranga@macdougall.bio | March 2024 08:54 PM | cmatranga@macdougall.bio | March 2024 08:25 PM | Gastrointestinal Stromal Tumors | June 2023 | American Society of Clinical Oncology Annual Meeting | |
| 10 | cmatranga@macdougall.bio | March 2024 08:55 PM | cmatranga@macdougall.bio | March 2024 08:25 PM | ErbB2 | April 2023 | American Association for Cancer Research Annual Meeting | |
| 11 | cmatranga@macdougall.bio | March 2024 08:55 PM | cmatranga@macdougall.bio | March 2024 08:26 PM | FGFR2 | April 2023 | American Association for Cancer Research Annual Meeting |

